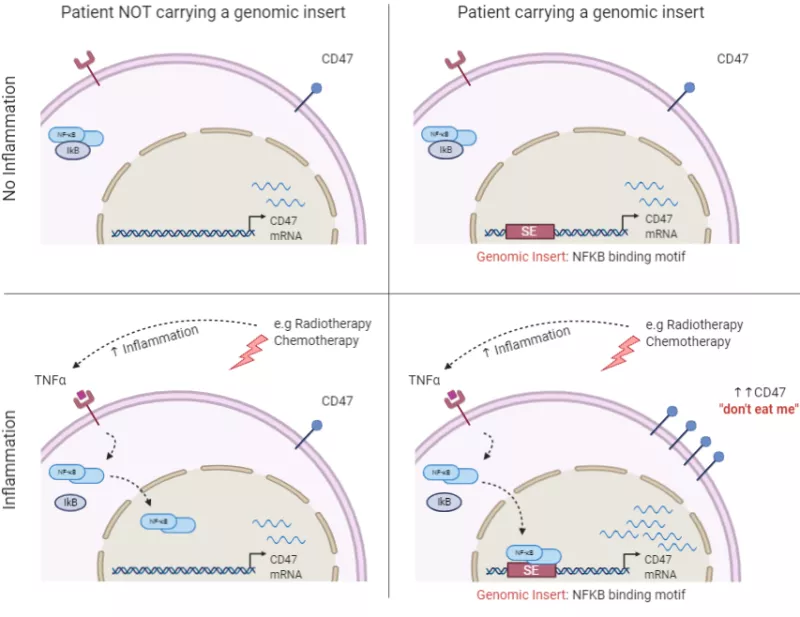
Investigate whether patient-specific genomic variants within super-enhancer signatures, activate an inflammation-driven immune escape program in certain cancer patients and provides resistance to radiotherapy. Upregulation of immune escape (e.g., CD47) genes can occur epigenetically and through super-enhancer (SE) loci, but we still don’t understand how they differ from patient to patient. For this project, we will determine in breast cancers whether a cohort of immune escape genes exemplified by CD47are dysregulated in response to a common TNF-NFKB inflammatory stimulus and by genomic inserts coding NFKB binding motifs identified prevalently in Western Europeans, Latinos and less in Asian populations. Furthermore, we will interrogate whether patients sensitive to inflammation are resistant to radiotherapy due to radiation-driven inflammation and its indirect effects on the upregulation of CD47 and other immune escape genes.
Address the effects of epigenetic memory in the aged immune system. Through this project we have the unique opportunity to interact with an international and multidisciplinary team of PIs (Dr. Hitoshi Takizawa from Kumamoto University and Dr. Ronald Huber from A*START). Ageing decreases hematopoietic stem cells (HSCs) and derivatives immune surveillance capacity, resulting in an increased in incidence of infection and cancer in the elderly. However, young HSCs can acquire epigenetic (inherited) and/or metabolic programs that provide memory to infection/inflammation history, to better respond to subsequent insults. Our goal is to test whether microorganism-derived agents, such as bacterial, viral, microbial components activate young HSC inheritable memory, thus improving HSC and their myeloid derivatives immune response. We hypothesize that young immuno-potent epigenetic programs can be imprinted onto aged hematopoietic stem cells by our method. This imprinting in turn, will be inherited by their myeloid derivatives improving their immunocompetence against infections or cancer as we age. This project was awarded a Catalyst Award from the US National Academy of Medicine and AMED, Healthy Longevity Global Grand Challenge.